Testing center GLOBALTEST
- ISO/IEC 17025:2017 Accreditation
- GMP Certificate (Medicinal products)
- GMP Certificate (Vet products)
About us
The services in the Testing Center GLOBALTEST аrе carried out in a specially designed and equipped laboratories, generally described as Chemical, Physic-chemical, Physical, Microbiological and DNA laboratories. The scope of activities extends to the following areas of study: pharmaceuticals, food and food supplements, cannabinoids containing products, cosmetics, household chemicals, essential oils, water, soil, waste, alcoholic and non-alcoholic beverages, toys, textiles, leather, packaging and packaging materials, lighters, silicate materials, plaster and more. TC Globaltest is a trusted partner in clinical trials. The Globaltest management, as well as the staff members, investigate, analyse and understand the current and the future customer needs. We strive to fulfil customer requirements and to exceed their expectations …
National phone
If you don’t know the internal extension number, please wait for the operator.
Sales & Marketing
+359 887 9594 06
Business hours
Monday - Friday
9.00 - 17.30
Saturday - Sunday
Closed
Please check out our services in the field of laboratory tests. In the “Forms” section you will find instructions for procurement
Services
Learn more about us and the latest developments around our activities.
News

Re-accreditation on ISO/IEC 17025 with extension of scope
Dear customers, As of August 29, 2024, the laboratory received its new Certificate of Accreditation with an extension of scope. The new accredited activity includes Testing of lighters in order to prove child safety using

Testing of medicinal products
Dear customers, you can familiarize yourself with the renewed services in the category “Testing medicinal products for human and veterinary medicine” You can view the section here

Catalog / price list of services
Dear customers, as of June 22, 2024, a new catalog/price list of services is in effect. The document is available in the Forms section. The IC GLOBALTEST team
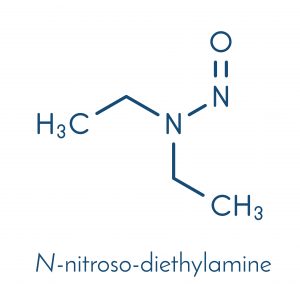
Information on N-nitrosamine impurities (N-nitrosamine impurities) in medicinal products
DEAR CLIENTS AND PARTNERS, Since 2018 there has been discussions of a problem related to the presence of N-Nitrosamine impurities in medicinal products containing chemically synthesised Active Pharmaceutical Ingredients (APIs). In this regard the EMA












